PSUR
PSURs are documents intended to provide an assessment of safety and performance for approved medical devices.
-
PSUR
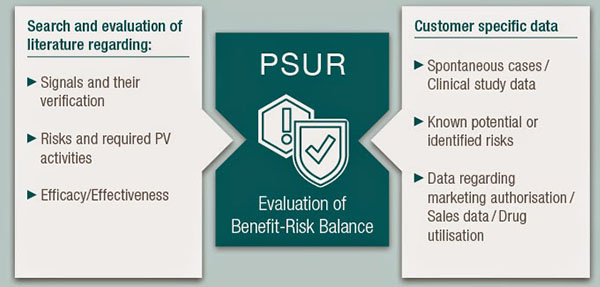
- PSUR is intended to provide an assessment of safety and performance of approved medical devices. The goal of the PSUR is to identify new risks to safety and performance, and to present comprehensive and critical analyses.
- Companies that manufacture medical devices rated Class IIa, Class IIb and Class III shall submit PSURs, submit a report on PMS data analysis to the certification authority, review it and upload it to the EUDAMED. PSURs should include conclusions about risks and benefit, PMCF reports, product sales and the nature of the population using the product, and the frequency of use of medical devices.
- Periods are updated annually for Class IIb and Class III devices, and Class IIa devices are performed at least once every two years.
PSUR services from DNA Technologies Pacific